Dear Colleague - September 1, 2024
September 1, 2024
Dear Colleague:
The South Carolina Department of Public Health (DPH) is pleased to announce the 5th annual PrEP Awareness Week. This annual statewide initiative aims to increase awareness among providers and the community regarding pre-exposure prophylaxis (PrEP), a pill or injectable that can help significantly decrease HIV transmission if a person is exposed to the virus. Although PrEP uptake, overall, is low in South Carolina, there has been a steady increase in prescriptions since the approval of PrEP in 2012.
Over the last five years, this annual event has grown significantly. DPH has increased partnerships with community partners and colleges and universities across the state to promote PrEP awareness and eliminate HIV stigma. Through these partnerships, DPH is able to deliver provider training to increase knowledge, develop culturally relevant messaging, and improve access to HIV testing. We invite medical providers, pharmacists, nurses, social workers, and health educators to join the daily webinars, or in-person symposium hosted in collaboration with the South Carolina HIV/AIDS Clinical Training Center, a partner of the Southeast AIDS Training and Education Center (SE AETC) to learn more about PrEP. Participants can earn up to seven FREE continuing medical, nursing, social work, or pharmacy education credits during PrEP week.
Additionally, these collaborations offer an opportunity to provide sexual health and PrEP resources to college students. Based on 2022 HIV surveillance data, individuals between the ages of 13-24 accounted for 56% of new HIV infections in the United States. Increasing HIV testing and PrEP awareness on college campuses will significantly help our collective goal of ending the HIV epidemic.
Visit the following webpages for more PrEP resources, and please share them widely:
For questions, contact PrEPMeSC@dph.sc.gov.
Sincerely,
Linda J. Bell, M.D.
Director, Health Programs Branch
State Epidemiologist
S.C. Dept. of Public Health
The South Carolina Department of Public Health's (DPH) HIV Pre-exposure Prophylaxis (PrEP) Statewide Plan and Guidance (The Plan) has been developed by DHEC as an element of the PrEPMeSC initiative. The Plan provides evidence-based guidance about procedures for increasing PrEP acceptance, PrEP prescribing, PrEP referrals, and diagnostic testing for participating providers.
The Plan will include but not be limited to the following subjects:
- Mission and purpose of the pre-exposure prophylaxis plan
- Clinical guidance for prescribing pre-exposure prophylaxis
- Referral guidance for clients who are screened as potential candidates
- Pre-screening guidance for clinicians to access client readiness
- Payment options for clients
The SC DPH STD/HIV and Viral Hepatitis division of the Bureau of Communicable Diseases and Prevention will be responsible for maintaining current guidance in The Plan and will serve as a source for technical assistance and education. The division will utilize CBA (capacity building assistance) to further enhance the capacity of funded and unfunded entities to effectively implement the pre-exposure prophylaxis plan
The STD/HIV and Viral Hepatitis division will provide basic procedures for engaging a client, scheduling an appointment, developing a referral process, and providing general procedures for required testing
The STD/HIV and Viral Hepatitis division will implement PrEP referral services in local health departments to address awareness and knowledge surrounding PrEP services. Clients can receive low-cost STI and HIV testing at local health departments. Social workers and disease intervention specialists (DIS) will also provide risk reduction and STI prevention counseling.
The goal of PrEP is to reduce the acquisition of HIV infection with its resulting morbidity, mortality, and cost to individuals and society. Therefore, clinicians initiating the provision of PrEP should:
- Prescribe medication regimens that are proven safe and effective for uninfected persons who meet recommended criteria to reduce their risk of HIV acquisition.
- Educate clients about the medications and the regimen to maximize their safe use.
- Provide support for medication adherence to help clients achieve and maintain protective levels of medication in their bodies.
- Provide HIV risk-reduction support and prevention services or service referrals to help clients minimize their exposure to HIV and other STIs.
- Provide effective contraception to women who are taking PrEP and who do not wish to become pregnant.
- Monitor clients to detect HIV infection, medication toxicities, and levels of risk behavior to make indicated changes in strategies to support clients long-term health.
What is Sexual Health?
The World Health Organization (2024)5 defines sexual health as the fundamental unit to overall health and well-being. Understanding the importance of sexual health is imperative because it empowers individuals to take charge of their reproductive health and emotional well-being.
Sexual health, when viewed affirmatively, requires a positive and respectful approach to sexuality and sexual relationships, as well as the possibility of having pleasurable and safe sexual experiences, free of coercion, discrimination, and violence.
Collecting a Sexual History
A sexual history is an opportunity for providers to learn about the client's sexual health. This involves more than just identifying their HIV/STI risk. Additionally, this is an opportunity for providers to share information to patients to assist them with safer sex practices, and to help them achieve their goals in their sexual health. Providers should emphasize the benefits versus the risks, which would be more effective in discussing risk reduction
| Components of a Sexual History (Five PS) 6.7 | |
|---|---|
| Partners | Are you currently having sex of any kind—so, oral, vaginal, or anal— with anyone? Are you having sex |
| Practices | What parts of your body are involved when you have sex? |
| Protection from STIs | If you use prevention tools, what methods do you use? How often do you use this/these method(s)? |
| Past History of STIs | Have you ever been tested for STIs and HIV? Would you like to be tested? Have you been diagnosed with STI in the past? When? |
| Pregnancy Intention | Are you or your partner using contraception or practicing any form of birth control? |
| *Pleasure, Problems andPride7 * | Are you have any difficulties or problems when you have sex, such as pain, vaginal dryness, low desire, not having an orgasm, or issues getting or maintaining an erection?” |
Recommended Vaccines
The following vaccinations can protect against infections transmitted through sexual contact and should be offered to eligible individuals.
- Hepatitis A
- Hepatitis B
- Human Papillomavirus (HPV)
- MPOX
Sexual Risk Reduction for Infectious Disease
After collecting the sexual history, the next step is risk reduction. This involves assisting the client with identifying less risky sexual behaviors if they are seeking to avoid infectious diseases.
Providers should, in an unbiased, nonjudgmental way:
- Encourage clients to use a new condom, consistently and correctly, for every sexual encounter during the entire act.
- Encourage clients to reduce the number of sexual partners.
- Encourage clients to limit or eliminate drug and alcohol use before and during sex.
- Encourage clients to discuss STI/HIV testing with partners.
Interventions
- Prescribe doxycycline post-exposure prophylaxis (DoxyPEP) for clients who wish to prevent bacterial STI.For more information on DoxyPEP, see Seattle-King County Guidelines.
- Offer rapid HIV and STI testing.
- Offer condoms and lube, and other harm reduction supplies as needed.
When screening, clients should be prioritized according to those who are at the highest risk. Clients who are seeking the following services should be assessed for possible PrEP services or referrals: HIV testing, STI screening, family planning, partner services or general inquiry. The priority populations, according to South Carolina epidemiological data of high HIV incidence and/or risk factors, should be assessed. This includes:
- Young persons aged 16-30 years
- Persons who have inconsistent condom use
- Persons who inject drugs (PWID)
- Persons who have tested positive for bacterial STI in the past 6 months.
It's recommended that a designated position, either social worker, disease intervention specialist, case manager, linkage coordinator, or nurse, who has, at a minimum, basic knowledge of pre-exposure prophylaxis provide PrEP navigation.
Positions appointed to conduct the screening may also refer to or familiarize themselves with the PrEPMeSC: Quick Guide (CR-012206), which includes basic PrEP information and questions to ask that will gauge readiness or interest in PrEP services.
- Clients who are screened (which includes taking a sexual history) and are interested in moving forward should:
- Be referred to a PrEP navigator or someone designated to assess potential PrEP clients for initial assessment.
- Be provided with PrEP counseling and resources.
- If PrEP services are not offered at your clinic, clients should be provided with information on the location of a PrEP provider.
- Ideally, clients would be given an appointment date and time for PrEP initiation. Multiple referrals and administrative hurdles decrease the uptake of PrEP.
- HIV Ab/Ag screening (4th generation preferred)
- HIV-1/HIV-2 testing and documented negative results are required prior to prescribing PrEP medications. For patient safety, HIV testing should be repeated at least every 3 months (before prescriptions are refilled or reissued). Note: Rapid tests that use oral fluid should not be used to screen for HIV infection when considering PrEP use because they can be less sensitive than blood. Clinicians should not accept patient-reported test results or documented anonymous test results
- HIV-1 RNA (viral load)
- HIV-1 RNA assay is recommended within one week of initiation (or collected at initiation) and follow-up due to the long duration of drug exposure with injectable PrEP. This is the most sensitive test to exclude acute HIV in clients receiving the injectable PrEP.
- Serum Creatinine
- Renal function screening should be assessed. Therefore, all persons considering PrEP should have a serum
creatinine test performed, and an eCrCL should be calculated utilizing the Cockcroft-Gault formula. Any
person with an eCrCl of <60 ml/min should not be prescribed Truvada® or TDF/FTC. If eCrCl <30 ml/min,
Descovy® or TAF/FTC is not recommended. Serum phosphorous is recommended in clients with chronic
kidney disease.- Follow-up testing should be performed every 6 months for clients > 50 years of age or who have a CrCl < 90
ml/m in at PrEP initiation. Annually for all other clients.
- Follow-up testing should be performed every 6 months for clients > 50 years of age or who have a CrCl < 90
- Renal function screening should be assessed. Therefore, all persons considering PrEP should have a serum
- Hepatitis B Ab/Ag Screening
- Vaccination against HBV is recommended for all adolescents and adults at substantial risk for HIV infection, especially for men who have sex with men (MSM). Therefore, HBV infection status should be documented prior to PrEP being prescribed. Those clients found to be HBsAg positive should be evaluated for possible treatment either by the clinician providing PrEP care or by linkage to an experienced HBV care provider.
- HBV infection is not a contraindication to PrEP use. Both TDF and FTC are active against HBV. HBV-mono-infected clients taking TDF or FTC, whether as PrEP or to treat HBV infection, who then stop these medications must be closely monitored for severe acute exacerbations of hepatitis B.
- Hepatitis C antibody
- Serologic testing for HCV is recommended for all persons starting PrEP. Persons who inject drugs or MSM are at higher risk for HCV infection and should be re-screened periodically based on their ongoing risk factors.
- Clients with active HCV infection (HCV RNA+ with or without anti-HCV seropositivity) should be evaluated for possible treatment because TDF/FTC does not treat HCV infection. When the clinician providing PrEP care is not able to provide HCV care, the patient should be linked to an experienced HCV care provider.
- Lipid Panel
- Higher rates of triglyceride elevation and weight gain was noted among F/TAF users. Persons being prescribed F/TAF should receive annual (every 12 months) triglyceride and cholesterol level monitoring.
- Sexually Transmitted Infections- Syphilis and GC/CT (3-point testing)
- Syphilis — Tests to screen for syphilis are recommended for all adults prescribed PrEP, both at initiation and follow-up screening visits.
- Gonorrhea/Chlamydia — Tests to screen for gonorrhea and chlamydia are recommended for all sexually active adults prescribed PrEP, both at initiation and follow-up screening visits. NAAT testing is preferred.
- Pregnancy testing, if applicable
- T/TDF is approved for PrEP use in pregnant and breastfeeding women. Data in the Antiretroviral Pregnancy Registry does not have any evidence of adverse effects among fetuses exposed to medication during pregnancy. Women who become pregnant while taking PrEP should seek prenatal care as soon as possible
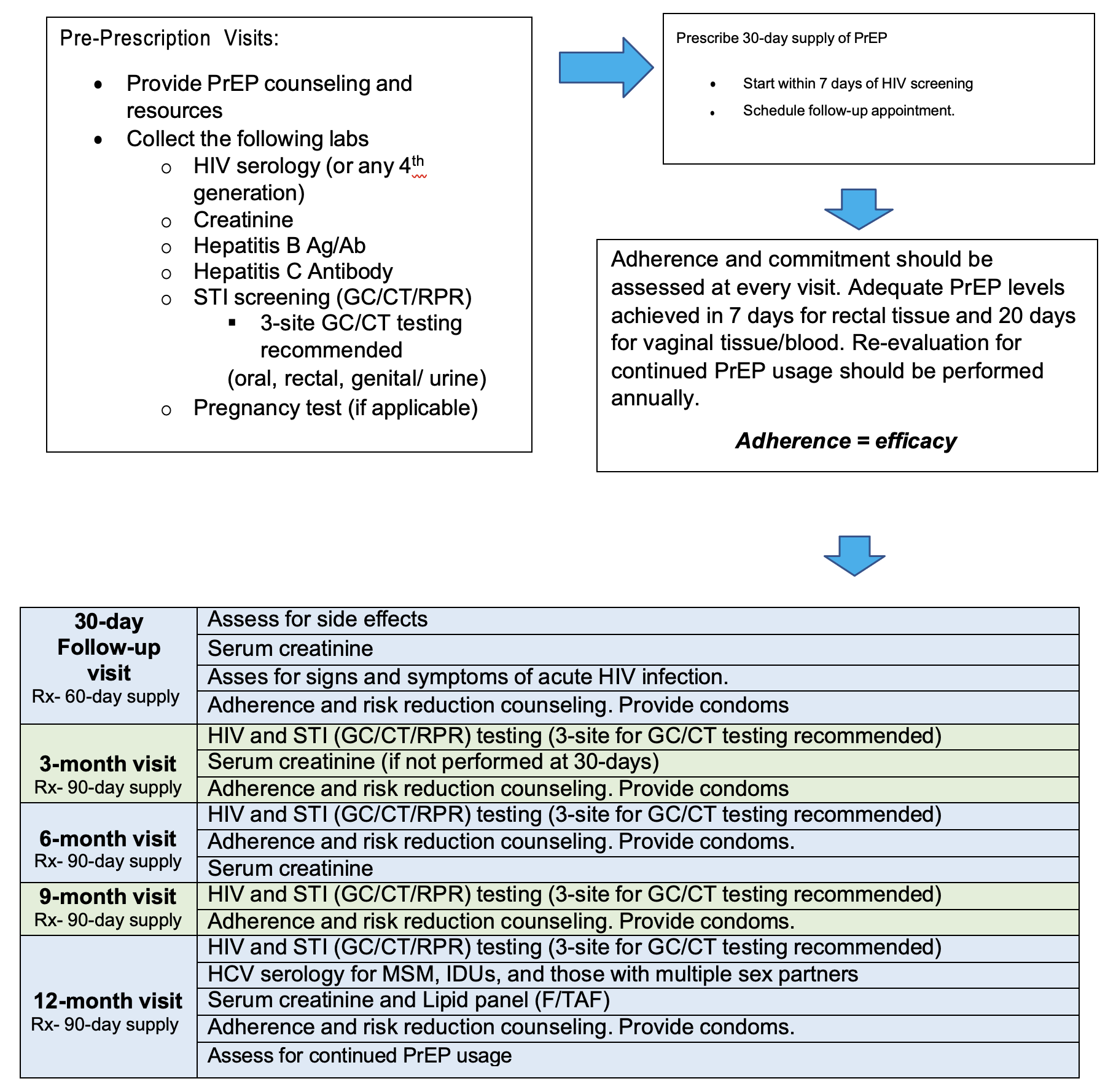
Prescription: FDA-approved medications (as of 1/1/2024)
Emtricitabine 200 mg and tenofovir disoproxil fumarate 300 mg; FTC/TDF (Truvada®) 1 tablet PO daily (30-day supply); up to 2 refills. Note: no refills with initiation. Patients should return to the clinic 1 month after initiation1,2.
Emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets; F/TAF (Descovy®) 1 tablet PO daily (30-day supply); up to 2 refills. Note: no refills with initiation. Patients should return to the clinic 1 month after initiation. Descovy® is not approved for cisgender women (women who identify with their assigned gender at birth) 1,3.
Cabotegravir 600 mg (Apretude) intramuscular injection (gluteal muscle) every 2 months 1,4.
*Optional* Cabotegravir 30 mg, 1 tablet PO daily for a 4-week lead-in prior to initial injection. Note: no refills with initiation. Patients should return to the clinic 1 month after initiation to receive injections 1,4.
Protection — maximum intracellular concentrations of TFV-DP (activated form of medication) achieved in the following durations1:
- Rectal tissue — 7 daily doses
- Cervicovaginal tissue — 20 daily doses
- Blood (peripheral blood mononuclear cells) — 7 daily doses
Data is not currently available for to estimate the maximum protection against HIV infection in F/TDF or F/TAF in penile tissue or injectable PrEP1
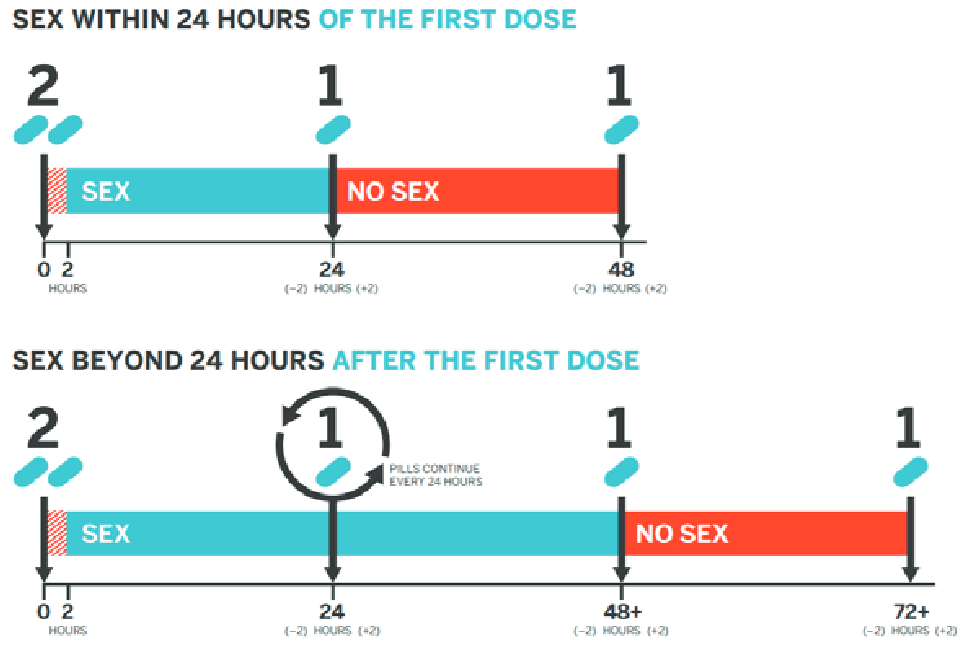
On-Demand (2-1-1) Dosing
This is a nondaily PrEP regimen that times oral F/TDF doses in relation to sexual intercourse events. While not an FDA-approved regimen, two clinical trials have demonstrated the efficacy of 2-1-1 dosing only with F/TDF and only for MSM.
Common Side Effects
- “Start-up syndrome” - usually resolve within the first month of taking PrEP. Discuss with client about the use of over-the-counter medication to assist with temporary side effects 1.
- Headache
- Nausea
- Abdominal discomfort
- Weigh loss reported with F/TDF use 2.
- Weight gain was reported with F/TAF use 1.
- Injection site reactions - Inform clients these reactions are common and transient. Clients can take over-the-counter medication prior to (1-2 hours) or after receiving injection or a apply a warm compress or heating pad to injection site for 15-20 minutes after injection 1.
- Pain
- Tenderness
- Induration
Adverse Effects
Oral Medications 2,3 — generally these medications are very well tolerated, and these adverse effects are all uncommon or rare.
- Renal Failure - FTC and TDF are eliminated by the kidneys. Renal impairment, including cases of acute renal failure and Fanconi syndrome associated with TDF 2,3.
- Decrease bone mineral density (BMD)- only observed in 3-4% of HIV-infected individuals taking medications with TDF. In clinical trials TDF was associated with slightly greater decreases in BMD and increases in biochemical markers of bone metabolism. Also, parathyroid hormone and 1,25 Vitamin D levels were also higher with TDF users. Unclear if it would be seen in HIV-uninfected individuals taking fewer antiretroviral meds 2,3.
- Severe Acute Exacerbations of Hepatitis B in clients with HBV.
- Lactic Acidosis and Severe Hepatomegaly.
- Serum Lipid changes reported with F/TAF 3.
Injection Medication
- Hepatotoxicity was reported in a limited number of individuals 4.
- Depressive Disorder (including depression, depressed mood, major depression, persistent depressive disorder, suicide ideation or attempt)
Additional Counseling
- Clients discontinuing the injection, should be informed about the long “tail” of gradually declining drug levels and the risk for developing a drug-resistant strain if HIV infection is acquired during that time 4.
DPH is working to improve data collection regarding PrEP uptake in the state. For those who are funded by DPH, you should use the following modalities to input data regarding PrEP:
- EvaluationWeb- Prevention sub recipients
- PrEP QA Monitoring Tool
Non-DPH-funded agencies (providers) can utilize the PrEP QA Monitoring tool to report PrEP data. Please submit the report by the 10th of each month to preventionreports@dph.sc.gov.
- DPH STD/HIV/VH Division: Nurse Practitioner, PrEP Program Manager, (803) 898-3939 Email address: prepmesc@dph.sc.gov.
- Clinician Consultation Center PrEPLine: 1-855-448-7737. Clinicians are available Monday through Friday, 9 AM - 8 PM EST
- University of South Carolina Telehealth: (803) 545-5402, http://schivtc.med.sc.edu/
- The Southeast AIDS Education& training Center Program: https://www.seaetc.com
| Coding For: | ICD-19 Codes | Description of Codes |
|---|---|---|
| Visit | Z29.81 | Encounter for HIV pre-exposure prophylaxis |
| Visit | Z01.812 | Encounter for preprocedural laboratory examination |
| Visit | Z20.6 | Contact with and (suspected) exposure to HIV |
| Visit | Z20.2 | Contact with and (suspected) exposure to infections with a predominantly sexual mode of transmission |
| Initial Labs | Z01.812 | Encounter for pre-procedural laboratory examination (Applicable to blood and urine tests prior to treatment or procedure) |
| Initial Labs | Z11.3 | Encounter for screening for infections with a predominantly sexual mode of transmission |
| Initial Labs | Z11.4 | Encounter for screening for HIV |
| Initial Labs | Z11.59 | Encounter for screening for other viral diseases |
| Initial Labs | Z72.89 | Other problems related to lifestyle (For Hepatitis C tests for patients insured through Medicare) |
| Initial Labs | Z20.5 | Contact with and (suspected) exposure to viral hepatitis |
| Subsequent Visits and Labs | Z51.81 | Encounter for therapeutic drug level monitoring |
| Subsequent Visits and Labs | Z79.899 | Other long-term (current) drug therapy |
| Subsequent Visits and Labs | Z20.6 | Contact with and (suspected) exposure to HIV |
| Subsequent Visits and Labs | Z20.2 | Contact with and (suspected) exposure to infections with a predominantly sexual mode of transmission |
| Subsequent Visits and Labs | Z20.5 | Contact with and (suspected) exposure to viral hepatitis |
PrEP is covered by most insurance plans and state Medicaid programs. The United States Preventive Services Task Force (USPSTF) gave HIV PrEP a grade recommendation of “A”, which means PrEP should be completely covered under almost all health insurance plans.
For uninsured or underinsured clients, there are programs available to assist with the cost of the medication, see below:
- Advancing Access: https://www.gileadadvancingaccess.com/
- Patient Advocate Foundation Co-pay Relief: https://copays.org/funds/hiv-aids-and-prevention/
- Ready, Set, PrEP (U.S. Department of Health and Human Services): https://www.getyourprep.com/
- Medication Assistance Programs: https://www.viivconnect.com/for-providers/viivconnect-programs/medications
- Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 Update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf. Published 2021.
- Truvada (emtricitabine and tenofovir disoproxil fumarate) [package insert] Foster City, CA: Gilead Sciences, Inc.; 2018. https://www.gilead.com/~/media/Files/pdfs/medicines/hiv/truvada/truvada_pi.pdf
- Descovy (emtricitabine and tenofovir alafenamide tablets) [package insert] Foster City, CA: Gilead Sciences, Inc.; revised 2019. https://www.gilead.com/-/media/files/pdfs/medicines/hiv/descovy/descovy_pi.pdf
- Apretude (cabotegravir extended-release injectable suspension) [package insert] Research Triangle Park, NC: Viiv Healthcare; 2021. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Apretude/pdf/APRETUDE-PI-PIL-IFU.PDF
- World Health Organization. (2024). Sexual health. World Health Organization. https://www.who.int/healthtopics/sexual-health#tab=tab_1
- Centers for Disease Control and Prevention. (2022). A guide to taking a sexual history - centers for disease control and ... Centers for Disease Control and Prevention. https://www.cdc.gov/std/treatment/sexualhistory.pdf
- National Coalition for Sexual Health. (2023). Sexual health questions to ask all patients: NCSH. National Coalition for Sexual Health. https://nationalcoalitionforsexualhealth.org/tools/for-healthcare-providers/ sexual-health-questions-to-ask-all-patients
Additional Resources:
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2020; vol. 33. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2022. Accessed [September 21, 2022].
- South Carolina Department of Public Health. South Carolina epidemiologic profile of HIV, AIDS, and sexually transmitted infections 2020. South Carolina Epidemiologic Profile of HIV, AIDS, and Sexually Transmitted Infections 2020. Accessed [September 21, 2022].
- Pre-Exposure Prophylaxis (PrEP)
- HIV PrEP Statewide Plan and Guidance
- Dear Colleague PrEP 2024
- CDC PrEP Guidelines
- HIV, STD, Hepatitis C Testing Locator & PrEP Providers
- HIV Self-Testing
- SC PrEP Providers
- PrEP FAQs (pdf)
- PrEP Patient Assistance
- CDC PrEP Site
- PrEP for Women
- PrEP Medication Info Sheet (pdf)
- Sexually Transmitted Diseases
- Tri-County SHAPE Resources
- PrEP Awareness Week

